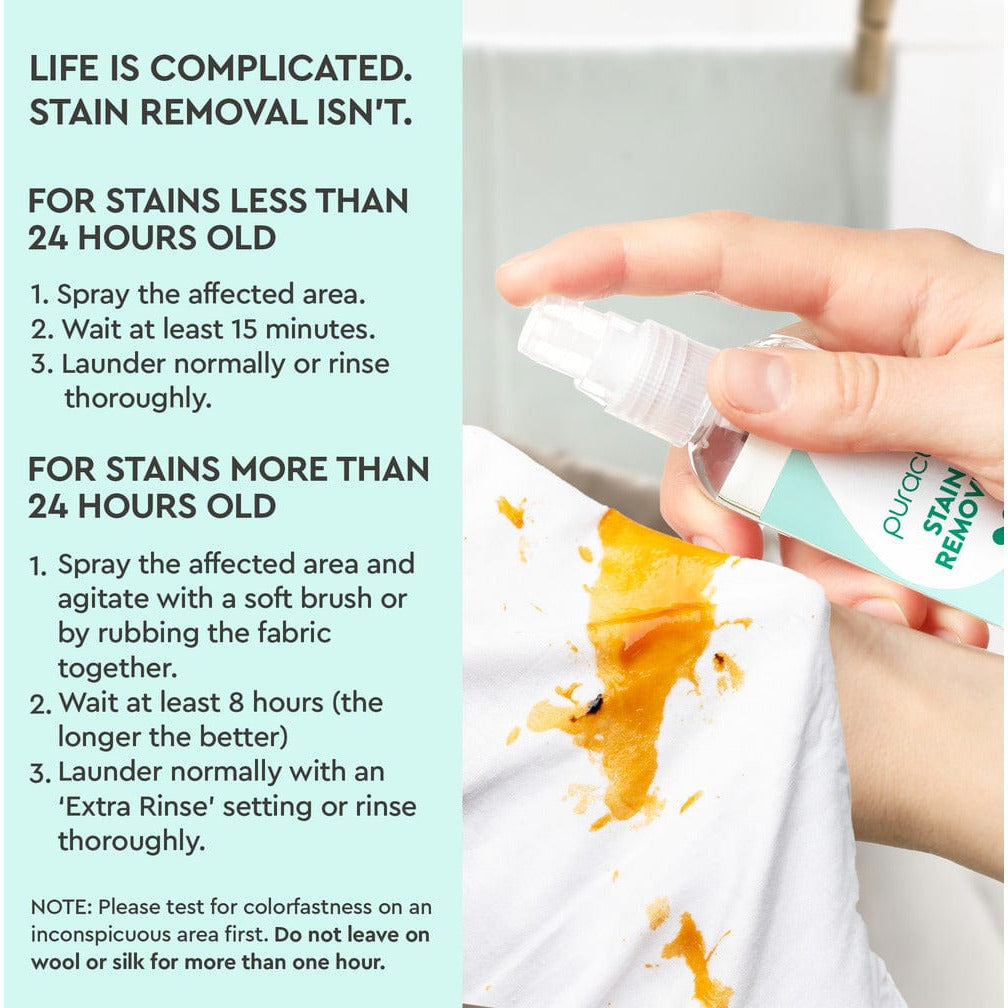
- Derived from: rice
- Pronunciation: (\ˈpan(t)-thē-nil\ hī-ˈdräk-sē-prō-pəl\ stē-ə-ˌr-dī-mō-nē-əm\ klȯr-ˌīd\)
- Type: Naturally-derived
What Is Panthenyl hydroxypropyl steardimonium chloride?
Panthenyl hydroxypropyl steardimonium chloride is a combination of panthenol, which is a plant-based, white crystalline powder derived from vitamin B5, and stearic acid, which is a white solid, often crystalline fatty acid that occurs naturally in animal and plant fats (typically coconut or palm oil) and is a major component of shea butter.[1,2,3]
What Does Panthenyl hydroxypropyl steardimonium chloride Do in Our products?
Panthenyl hydroxypropyl steardimonium chloride is a hair conditioner that helps keep hair soft and smooth; it also fights static.[4] It is found in dozens of personal care products, including shampoo, styling gel and mousse, hair color, hair spray and other items.[5]
Why Puracy Uses Panthenyl hydroxypropyl steardimonium chloride
We use panthenyl hydroxypropyl steardimonium chloride in several of our products as a hair conditioner. The Cosmetics Ingredients Review has deemed panthenol (vitamin B5 — a primary component of the ingredient) as safe in cosmetics.[9] Research also shows that vitamin B-5 is not a strong skin irritant.[10,11,12,13,14,15,16,17] Whole Foods has deemed stearic acid — another component — acceptable for body care products, and studies show it is not a skin irritant.[18,19,20,21,22] In addition, the Cosmetics Ingredient Review has deemed stearic acid safe for use in cosmetics, and the FDA has deemed it Generally Recognized As Safe (GRAS).[23,24,25,26]
How Panthenyl hydroxypropyl steardimonium chloride Is Made
Panthenyl hydroxypropyl steardimonium chloride is a derivative of panthenol, which is made by combining 3-amino-1-propanolamine with the lactone of 2,4-dihydrocy-3,3-dimethyl butyric acid.[6] Pantothenic acid is made by condensing 3-aminopropanoic acid with the lactone of 2,4-dihydroxy-3,3-dimethyl butyric acid.[7] Panthenol can also be extracted via benzyl alcohol from an ammonium sulfate aqueous sample and then purified; in addition, it can be separated from sugar.[8]
Sources
[1] Proctor, R. and Thomsen, L. Veganissimo A to Z: Comprehensive Guide to Identifying and Avoiding Ingredients of Animal Origin in Everyday Products. (2013) Workman Publishing
[2] American Cleaning Institute
[3] U.S. National Library of Medicine
[4] Environmental Working Group
[5] Environmental Working Group
[6] Kilic, A., Cicek, H. "Formulation for Topical Wound Treatment"
[7] Personal Care Council
[8] Gyorgy, P., Pearson, W.N., eds., The Vitamins: Chemistry, Physiology, Pathology, Methods. (2016) Elsevier
[9] Personal Care Council
[10] CTFA. (No date). Eye and skin irritation testing in rabbits with DL-Panthenol and Dexpanthenol. CTFA Code No. 3-8-2
[11] CTFA. (No date). Skin irritation study of a product containing 0.5 percent Panthenol. CTFA Code No. 3-8-21
[12] CTFA. (1981). Acute dermal and ocular irritation testing of a mascara containing 0.5 percent Panthenol. CTFA Code No. 3-8-16
[13] CTFA. (1983). Four-day minicumulative human skin irritation study with a product containing 0.5 percent Panthenol. CTFA Code No. 3-8-22
[14] Hill Top Research, Inc. (1983). Report of a human skin test of cumulative irritation. CTFA Code No. 3-8-4
[15] Leo Winter Associates, Inc. (No date). Human repeated insult patch testing of a product containing 0.5 percent Panthenol. CTFA Code No. 3-8-10
[16] CTFA. (No date). Human repeated insult patch testing of a mascara composite containing 0.5 percent Panthenol. CTFA Code No. 3-8-19
[17] Leo Winter Associates, Inc. (1974). Human repeated insult patch testing of a cream containing 0.5 percent Panthenol. CTFA Code No. 3-8-12
[18] Butcher, E.O. (1951). “The effects of application of various substances in the epidermis of the rat.” Journal of Investigative Dermatology. 16, 88
[19] CTFA. (Aug. 14, 1974). Submission of unpublished data. (3-3-10). Safety evaluation of 2.0 percent Stearic Acid in 01 B/62568-)(6020). Four-week subacute dermal toxicity study in rabbits
[20] CTFA. (Aug. 14, 1974). Submission of unpublished data (3-3-16). Four-week subacute dermal toxicity study in rabbits: 2.0 percent Stearic Acid
[21] CTFA. (July 11, 1980) Submission of unpublished data. (3-3-21). Study project 0135. The safety evaluation of two sun protection products and one facial skin care product. Thirteen-week subchronic dermal toxicity study in albino female rats on 5.0 percent Stearic Acid
[22] Whole Foods Market
[23] CTFA. (Aug. 18, 1982). Submission of unpublished data. (3-3-15). Study project 0191. The safety evaluation of three make-up products: 2.4 percent Stearic Acid in 036/18218-12. Thirteen-week subchronic dermal toxicity study using female albino rats
[24] Personal Care Council
[25] Food and Drug Administration
[26] Food and Drug Administration