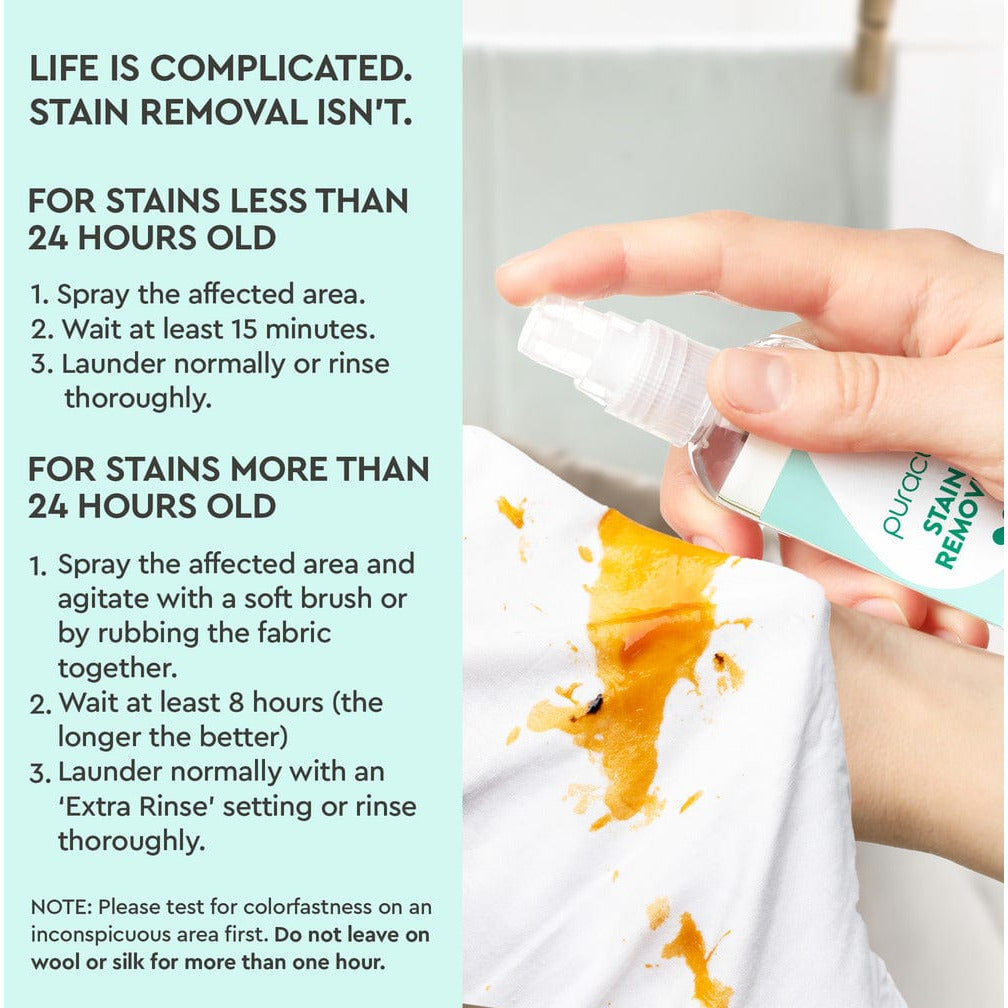
Learn all about c9-c11 alcohol ethoxylate — how it's made, and why Puracy uses this ingredient in our products.
- Derived from: Coconut
- Pronunciation: (\ˈal-kə-ˌhȯl\ \ˈetho-zī-lāt\)
- Type: Naturally-derived
- Other names: C9-11 Pareth
What Is C9-C11 Alcohol Ethoxylate?

C9-C11 alcohol ethoxylate (also called C9-11 Pareth) is a colorless liquid derived from palm kernel or coconut oil. This group of surfactants is often used in wastewater treatment and its hydrophobic properties make it an effective wetting agent.[1,2]
What Does C9-C11 Alcohol Ethoxylate Do?

C9-C11 alcohol ethoxylate is an emulsifier and a surfactant that breaks the surface tension of water, allowing things to become clean. It can be found in detergents and personal care products, such as styling mousse, hair gel, and other styling products.[3,4]
How C9-C11 Alcohol Ethoxylate Is Made

Commercial alcohol ethoxylate is a mixture of several homologues of varying carbon chain length and degree of ethoxylation. Homologues that are not ethoxylated are also called aliphatic alcohols or simply fatty alcohols.[5]
Primary alcohol ethoxylates are made via ethoxylation of primary alcohols with ethylene oxide, using base catalyzed reaction with potassium or sodium hydroxide.
Neutralization with an acid such as acetic or phosphoric acid then occurs. Most commercial alcohol ethoxylates are made and delivered as solids, pastes, or solutions.[6]
Why Puracy Uses C9-C11 Alcohol Ethoxylate
We use C9-C11 alcohol ethoxylate as an alternative to ammonia. It has the unique ability to clean a surface and then evaporate without leaving a residue (which is especially helpful when cleaning glass, stainless steel and similar surfaces).
Although ethoxylated alcohols may experience 1,4 dioxane contamination as a byproduct of the production process, the EPA considers it safe to consume water with 4 ppm of 1,4 dioxane for one day or 0.4 ppm of 1,4 dioxane for 10 days.[7] A study of workers exposed to 0.006–14.3 ppm 1,4-dioxane for an average of 25 years found no evidence of liver or kidney disease or any other clinical effects, and another study found no differences between observed and expected incidences of cancer among workers at a manufacturing and processing facility.[8]
Puracy has signed certifications from our ingredient providers that show no more than 2 ppm of 1,4 dioxane in our most concentrated finished product. We only use these ingredients in cleaners, which are rinsed or wiped away with a cloth rather than applied to the skin.
Is C9-C11 Alcohol Ethoxylate safe?

As an ingredient commonly found in household cleaning products, it’s important to know the nature of C9-C11 alcohol ethoxylate to human health and its environmental impact.
It is generally safe in household cleaning products at recommended concentrations. It can be found in laundry detergents, dishwashing detergents, all-purpose cleaners, glass cleaners, and floor cleaners.
Safety to Human Health
C9-C11 alcohol ethoxylate is considered safe to human beings due to its low toxicity. While it’s safe to use as an ingredient in your household cleaners, it’s still important to wear gloves when you’re using it and avoid contact with the skin and eyes. Avoid ingesting this product as it may cause discomfort.
Prolonged inhalation may cause irritation of nose, throat, and airways. It may also cause redness and irritation after lengthy exposure at high concentrations.
Safety to the Environment
C9-C11 alcohol ethoxylate is biodegradable and can go straight down the drain as part of laundry water or other cleaning residuals. The European Chemicals Agency (ECHA) considers C9-C11 as a toxic substance to marine life.
It has no potential to bioaccumulate, which is used to determine toxicity when an element concentration is taken in and stored faster than it can be released.
Sources
[1] HazMap
[2] Human & Environmental Risk Assessment
[3] Environmental Working Group
[4] Ash, M. and Ash, I. Handbook of Green Chemicals Synapse Info Resources (2004):677
[5] Sanderson, H. et al., "Occurrence and risk screening of alcohol ethoxylate surfactants in three U.S. river sediments associated with wastewater treatment plants," Science of the Total Environment Volumes 463–464, 1 October 2013, Pages 600–610
[6] Human & Environmental Risk Assessment on ingredients of European household cleaning products
[7] Agency for Toxic Substances and Disease Registry
[8] Agency for Toxic Substances and Disease Registry